기업의 더 효율적인 소프트웨어
선택을 위한 17년 지원 경험
Greenlight Guru
Greenlight Guru은(는) 무엇인가요?
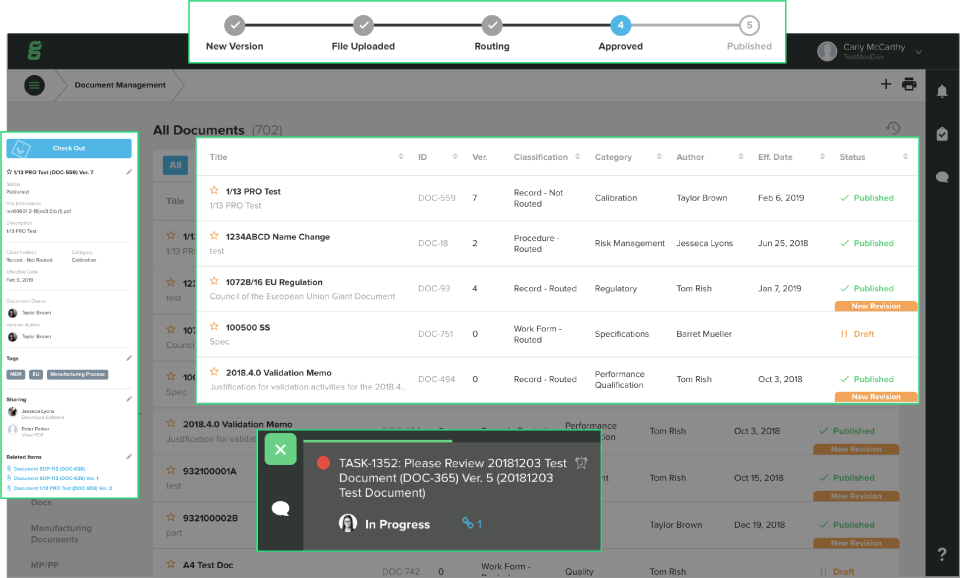
Greenlight Guru는 의료 기기 회사를 위해 특별히 설계된 유일한 품질 관리 플랫폼입니다.
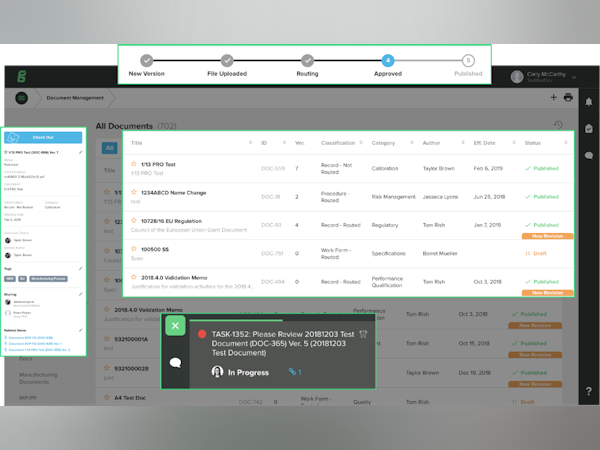
이 플랫폼은 회사가 더 안전한 제품을 더 빠르게 출시하도록 지원하고, FDA 및 ISO 규정 준수를 간소화하고, CAPA, 위험, 감사, 문서 제어, 교육, 디자인 제어 등의 모든 품질 프로세스 관리를 연결하여 단일 정보원을 제공합니다.
전 세계 기기 제조업체들이 오래된 종이 기반의 범용 레거시 품질을 대체하고 있습니다.
Greenlight Guru은(는) 누가 사용하나요?
Greenlight Guru는 제품 출시에 필요한 시간과 비용을 절약하길 원하는 의료 기기 회사를 위한 완벽한 솔루션입니다. 당사 고객에는 QA/RA 역할, 엔지니어 및 임원진이 모두 포함되어 있습니다.
Greenlight Guru에 대해 확실하지 않으세요?
인기 있는 대안 제품과 비교

Greenlight Guru
Greenlight Guru 리뷰

A god-send of a QMS
주석: As a new medtech company with many different product and differing product regulations, we are pleased to have Quality Management as one of our easiest tasks - which is rare to say. The team is fantastic and abundantly helpful, the system is quick to learn and easy to use, and the support you get standing the system up from scratch if you don't already have a system in place is great. We needed Quality "handled" so we could focus on the trickier parts of our company - Greenlight Guru was and is that perfect solution.
장점:
With GG, it is structured and organized to not just allow compliance, it promotes compliance - meaning using the software as it is built easily documents for product design/dev and ongoing quality req'ts. It's easy to use and navigate, quick to learn even for lay people, and makes our QMS regulatory headache-free.
단점:
The team keeps making improvements, and I'm looking forward to a bit of a better structure to the large document repository. It is searchable, tagable, and sortable easily, but there are a few other ways that they could organize things so you can find something quickly.
Greenlight Guru 응답
6년 전
Hi Sarah, The team at GG greatly values your feedback and we appreciate you taking the time share your experience working with our software and team. Glad to hear you are excited about the upcoming enhancements to Advanced Document Management with with debut of Document Views in our December 2018 release. We strive to simplify quality management for medical device companies of all sizes, and welcome any future feedback or enhancements ideas.
Truly Turnkey, Great for new companies
주석: Greenlight.Guru has allowed us to, from a very young, inexperienced stage, build from scratch an easy to use and comprehensive quality system that has passed external audit with ease. The customer support, user interface, and turnkey solution offered by Greenlight.Guru has exceptionally useful for us, and been the foundation for Quality Management at our company.
장점:
Using Greenlight.Guru, we were able to build a quality system from scratch quickly that met our needs without the headache of complicated set-up and configuration. This tool enabled us to go from nothing to passing our first audit with confidence and ease. Great tools to link design and risk with documents and approvals. The customer service was also great, and Greenlight helped build our knowledge of Medical Device Quality at the beginning of our company's life, allowing many of us to rise from inexperience to competence to near mastery of quality systems. Greenlight further offers an unparalleled user interface and intuitive tools that make using it a cinch for almost all employees at our company.
단점:
Understanding best practices can be a bit challenging and time consuming given that there is one standard offering for all customers and customization is intentionally not available. Being a new company, Greenlight has grown with us. Some features that would have been nice to have earlier were developed and pushed during our use of the software, though it is clear that the product is maturing to meet the needs of its customers without sacrificing the ease of use.
More functionality preferred
주석:
Overall the software is easy to use, but there are a few functionality that we wish were there, specifically:
- parallel approvers
- ability to attach redlines as part of routing (and then ability to publish clean copy); now it forces you to review redlines offline (not great since it gets lost in people's email, etc.)
- ability to reassign who can publish the document (if person who routed it is out and doc control/quality should be able to publish for them).
- ability to turn on email notifications if you have something in your inbox
장점:
online
단점:
very limited functionality
Greenlight Guru 응답
6년 전
Hello, Dieu. Thank you for your feedback. We heard you, loud and clear. As a result of your feedback, we've added parallel routing and the ability to include redlines and mark-ups to review and approval workflows. Additionally, we've since enabled user level email notification preferences for you to better control the types of messages you'd like to receive via email.
Greenlight is the right choice for eQMS
주석: We evaluated multiple eQMS platforms, we went with Greenlight due to functionality, ability to scale as we grow, and the extensive / pre-existing documentation that we could leverage and tailor to our needs.
장점:
Building a maintaining a QMS system that is FDA compliant is far too complicated and expensive for an early stage startup. Greenlight gives you the tools and platform to follow a pre-determined process that allows you to get up and running quick, while also having the confidence that you are meeting required regulations. Team has always been super helpful with great customer support.
단점:
eQMS can be complicated with lots of jargon. It does take a sustained push to become aquatinted with terminology and incorporate into you daily workflows.
Only eQMS geared towards medical devices
주석: Great experience! Customer service is quick to respond and there is a help centre and greenlight academy with tutorials for quick reference. The traceability between design controls and risk elements is in itself worth it.
장점:
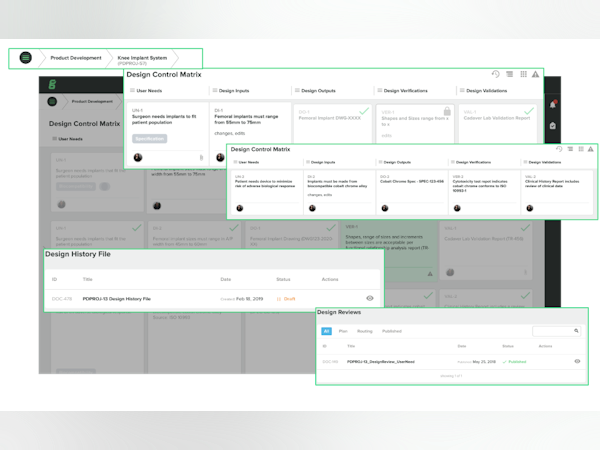
Wow so many things - selecting/modifying roles/permissions, creating teams, personalizing profiles, tagging documents, grouping documents, adding/uploading w/ w/out a change order, creating/activating training events, tracking training events' progress/completion, automating/completing quality events (CAPAs, nonconformance, audits and customer feedback), adding/modifying/tracing design controls i.e., user needs, design inputs/design outputs, V & V and finally adding/modifying/tracing risk items and aligning them to the project. Customer service is quick to respond and Greenlight Guru is always taking suggestions for improvement, they have even implemented some.
단점:
Just some suggestions for better functionality - ability to edit tags, ability to edit training events once activated or in the case the documents assigned to a training event changes, more user friendly quality event workspace, ability to easily change between full and lite users and vice versa.
My GG Experience
장점:
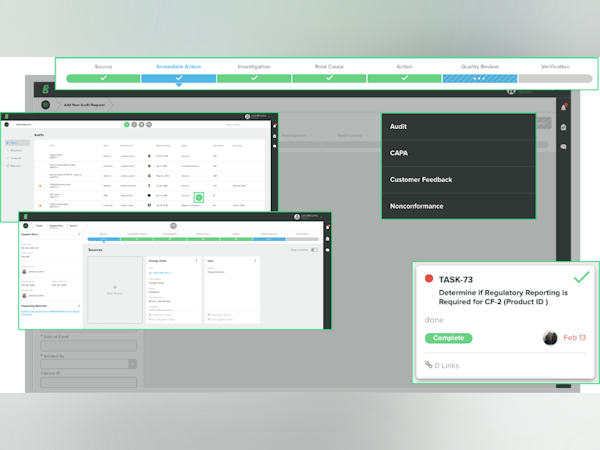
GG is hands-down the most user-friendly s/w I have ever worked with. The GG team has truly created simple easy-to-use workflows, while still offering complex functionality that conforms to the rigors of ISO 13485 standards. I love how you can link tasks, documents, investigations, and CAPAs. I personally love their DMS and Customer Feedback workflows. They're easy on the eyes, tell the whole story and allow for easy routing and approving to key stakeholders.
단점:
GG's data analytics aren't very helpful and often inaccurate. The data they're tracking is rudimentary and seems to an after thought by the design team. Additionally, there is no reporting functionality in their Customer Complaints workflow. For example, if you're attempting to run a trend report for common root causes related to certain events, the s/w doesn't allow it. Therefore, I have to track everything outside of GG, especially for trend reports. Trend reporting and tracking is critical in any regulated industry, so I'm not sure why GG didn't think of this
Great software to implement and maintain a medical device QMS
주석: Overall experience is great. As a small medical device company, I can't imagine keeping up with our QMS requirements without a software platform like Greenlight Guru.
장점:
The design trace matrix makes the development process very straight forward and easy to use. The ability to link anything to each DTM component, and to link the DTM to the risk management module is great as well. The document management and routing/approval features are also a great tool. We've been able to implement digital versions of most of our work forms, without the need to use third party document signature programs.
단점:
Sometimes there is a bit of a learning curve for the various modules that they release. It would be nice if a help/guide button was available for each to walk through the module step by step.
Greenlight Guru 응답
6년 전
Hey Jason: Thank you for the positive comments and feedback. Glad to hear that the ability to Link Anything is positively impacting your Design Control/Risk efforts and that built in workflows with eSignatures are simplifying processes. As it relates to your feedback on getting up to speed on how to use new functionality, know that we've heard you and are actively working on a solution to help you get up to speed on how to use new functionality and what's changed in app that I believe you will be pleased with. Don't ever hesitate to share additional feedback like this with us by phone, chat, or email at customer.success@greenlight.guru
Makes Implementing a Medical Device eQMS Easy
주석: I work for a small medical device startup, and we've used Greenlight Guru to set up our quality management system from day one. They've made complying with ISO and FDA standards for quality management straightforward, they keep adding helpful functionality to the system, and I look forward to continuing to work with them!
장점:
Greenlight Guru has a very intuitive interface that lets me manage document changes and design control from the same dashboard. I can sit down and set up a change order to release and version multiple SOPs in an hour with a single set of approvals, which really streamlines my document control workflow. It's also perfect for remote work, since it automatically captures electronic signatures and time stamps, and it's already set up to ping users by email when it's time for them to complete a task. And I must commend their customer support team - they are an admirably responsive and helpful team of people who add so much value to the experience with this company.
단점:
Inputting a large number of documents into the system at a time can be fairly tedious. Some kind of mass upload function would be helpful at some point. I would appreciate an integrated training workspace as well, potentially tied to the document approval process.
Greenlight Guru for OtoNexus
주석: I am a consultant, working with OtoNexus, an early-stage medical device company, on the implementation of their Quality Management System (QMS). We advised them to consider deploying Greenlight Guru for this purpose, and they are in the initial stages of doing so. The Document Management features of the software appear to be thorough, well thought out and useable. Obviously, the flexible routing and detailed history features for document review and sign-off, and compliance with Part 11, are very helpful and key to the success of any medical device company's QMS. The tutorial on the Product Development module, which is based on 14971 risk management principles, seems like it will be an excellent tool. Unfortunately, the client is a bit behind in utilizing it, and wants some other project management features implemented that, frankly, are a bit beyond the scope of this type of software tool. Customer support in general has been excellent. We have noted, however, that when product feedback is given, although the response to a question or issue is timely, there is often a defensive tone that comes across. An example of this was when there was a change to the software, which caused a conflict with login using a browser that had worked fine previously. Yet, we were not notified of this change, and once we found the issue, were told to update our browser. This software, like any other, is imperfect, and can and should be continuously improved. Overall, my initial impressions are that this is an excellent software tool for implementing a QMS at a medical device company.
Greenlight Guru 응답
6년 전
Hi Grant, thank you for taking the time to write your review of greenlight.guru. You input, and the feedback from all our customers is very important to the continuous improvement of our software. Since your original feedback on 12/1/16, we've evolved our internal release processes and documentation in order to assure changes such as this are better communicated with customers. Thanks again for your feedback and partnership.
GreenLight is the best product development software
장점:
GreenLight team members are very responsive to concerns. The software itself is essential for any small-medium company hoping to get through the product development process and receive FDA clearance/approval.
단점:
I wish there was a training module. Training currently uses up a good amount of our time.
Medical device startup's experience
주석: greenlight.guru is the perfect software solution for a medical device startup company looking to get a quality management system, compliant with both the FDA's and ISO's requirements, up and running. It also holds your hand through the design control and risk management process, which is especially valuable for a company without much experience going through these processes. We didn't need to hire a quality manager because of how helpful this software is, a fact that alone makes it worth the price.
장점:
The structure of the design control and risk management modules guide you through the process of documenting both. Especially great for someone who's never gone through these processes before.
단점:
While it contains the core of what's necessary for a QMS, it lacks a lot of functionality that a larger business would need, making it likely that we'll outgrow it quickly.
Greenlight Guru 응답
8년 전
Hi Matthew, we appreciate you taking the time to write your review of our software. That's great to hear greenlight.guru has enabled you to delay the hire of a full time quality manager. Per your comment regarding the feature set needed for a larger business, we'd love to hear your specific recommendations if you have them so that we can take them into account as we continue to execute on our roadmap. You can reach out to us and provide your feedback directly to customer.success@greenlight.guru. To date, we have several larger medical device companies with multiple products and hundreds of employees that are successful using the platform.
Great product, continuously improving!
주석: We needed an eQMS system, and a method of tracking our project progress. Greenlight.Guru does that in a fluid, comprehensive manner - making my life far easier.
장점:
Greenlight.guru successfully ties risk analysis to design controls, providing a method of reviewing all of the design or component by component. All of the associated documents can be linked to Design items, weaving in the entire story behind a particular product. I have not found a similar product, and have never seen a more dedicated team. Issues that I have encountered, they resolve in a thoughtful, forward-thinking manner.
단점:
In the early usage, there were difficulties finding the documents I wanted. They have recently added the linking functionality on the document side, which allow related items to be tied to each other.
Greenlight Guru 응답
6년 전
Hey Stephen, Thank you for the kind feedback. The team at GG is dedicated to assuring the success of our customers. Glad to hear the linking functionality is benefiting your efforts. Additionally, I think you'll be pleased with upcoming efforts we are making to further simplify document management starting with the debut of Document Views in our December release. Keep the feedback coming.
Recommendation for Greenlight Guru as your medical device eQMS solution
주석: Ease of integration of design control with risk management is a breeze. No more tedious xcell spreadsheets!!
장점:
I like that this medical device software is built by medical device professionals and not just SW developers who don't even know what a medical device is! this SW is built to match the processes I have been doing now for over 35 years - document control, design control, capa, internal audits, etc. Both modules GO and GROW are on spot to meet the regulatory requirements.
단점:
As with any SW we have to be methodical in our naming conventions as we load documents. Its all about setting rules as a company so that SMART naming is followed which makes the search mechanisms more effective.
Greenlight Guru 응답
6년 전
Hi, Penny. Really appreciate your feedback and comments. Did you know that all documents are assigned a unique ID when added now? Yes, there is still a need to apply some intelligence with document naming and description. Those attributes (regardless of system) are key.
Sleek Medical Device QMS Software with a great Team!
장점:
The system workflow is intuitive and easy-to-use right out of the box. We currently utilize the document management system and the module allows for quick uploads, document organization and ease of finding documents. The Greenlight Guru team behind the software works with you to meet your business objectives. Additionally, the have a strong customer focus and really listen to the voice of the customer.
단점:
The search function can be a struggle at times; would really like the opportunity to filter further.
Greenlight Guru 응답
6년 전
Hi Tonia - Thank you for sharing the positive feedback with our team. Our team is happy to hear that you value the turn-key nature of our solution for medical device companies. We strive to simplify quality for our customers and removing the burden of assuring documentation is compliant to regulatory standards is an essential piece in that effort. Please know that we hear your feedback regarding the need to find things faster within the system. I believe that you'll find the debut of Document Views in our December 2018 to be a starting point in helping you achieve this as we further enhance search and filtering functionality.
A viable mvp but not really ready for commercial needs
주석:
We have been using Greenlight Guru now for a few months and I note the following concerns:
1) There is no undo button - once editing in a screen if you accidentally change something there is no way to return to the original state - clicking the close button will save ANY change you make even incidental ones.
2) Loading time is ridiculous. Our software currently has fewer than 300 requirements total and loading can take a few minutes.
3) Traceability for risk doesn't yet work properly in terms of tracing right through to V & V
4) Workflow could be dramatically improved - so many clicks are required to create each requirement.
5) Sometimes you will create a requirement and it does not show up in your list until you refresh the whole list - which led to a couple of duplicate requirements being created and these then can't be deleted - only obsoleted.
6) Document signing order created huge headaches for us organizationally in efficiently getting documents routed and signed.
7) Users who were in the application at the time of document routing received no notification a document was waiting for them.
8) There should be a way to check all validation and verification test cases within a given protocol off at once. Individually checking them off as passed is a make-work exercise.
9) Search feature is totally brutal and sometimes will not find something as simple as the DI title (e.g. searching for DI-159 which exists does not necessarily return DI-159).
10) When switching between projects - window did not always update content - leading to editing the wrong requirements
11) No way to sort requirements except through tagging- no way to view a short list of requirements only with a given tag reliably since search function can't be relied on.
장점:
Feature to read across related user needs, di, do and testing is a good idea. Printed matrix document looks nice.
단점:
So much clicking to accomplish needed tasks; not all features work as needed or expected.
Greenlight Guru 응답
6년 전
Hi, Megan. Thank you for your candid and detailed feedback. We took your feedback seriously and made updates to the Greenlight Guru software to address many of the issues you mentioned.
Great and Developing Document & Design Controls
주석: GreenLight.Guru (GLG) is a terrific tool that solves multiple issues - design controls, document controls, and compliance. Perhaps just as valuable is an unending stream of blog posts and educational articles from the GLG staff. Their goal is to help users of their software (and, frankly, just about everyone else) adopt best practices in medical device design and maintain compliance during the product development process and beyond. The system is not very intimidating, but still requires some sophistication to use properly. That being said, it enables a single person to do the work of several people, which is extremely efficient for a small or start-up company. My current "complaint" is that our GLG installation does not send email notifications about tasks or documents to review. This would be very beneficial.
장점:
Easily allows for document control in a compliant system for medical device development
단점:
I like traditional interfaces with document lists that look like a "Window" rather than highly formatted presentations. Give me simple presentation.
Greenlight Guru 응답
6년 전
Hi Michael, our team appreciates you taking the time to review our software and provide your feedback. I'm happy to share that functionality for automated e-mail notifications are now available. Additionally, individual users can modify notifications to their liking. Please don't hesitate to provide additional feedback. Our team is actively listening to the evolving needs of our customers as we develop new functionality.
Wrong kind of software for our demand so far
주석:
Three main deficits: First there is no strukture for the data we save in Greenlight like direktorys, subdirektorys, a kind of tree... Colours... The only method to find data in the large pool is the serch funktion. This is useless if you don´t know the right name of what you are looking for.
Second deficit: We were told that data exchange with other systems is not possible. If that is really true I would immediately stop this software, but keep cool, I am far away from beeig responible.
Third deficit: Though the speed of the operations improved, it is still not acceptable. Important to know would be if the internet-connection we have at invendo is sufficient and reliable.
Positive: I have no doubt that you are able to program every kind of user interface we need. And I am very happy that you are really interested in my opinion. Feel free to ask me specific Questions.
Regards, Till Hoffmann
Greenlight Guru 응답
6년 전
Hi, Till. Thank you for your candid and honest feedback. Since your comments, Greenlight Guru has made quite a few product updates. Document management has been enhanced with additional data attributes and ability to search, filter, and sort information more easily. We continue to work on data exchange and have had some conversations with your colleagues on this. Speed and performance are constantly monitored and being updated to ensure you get the information you need as timely as possible. Keep the feedback coming!
Initial Review
주석: To date, GG has been an excellent partner in the implementation of an otherwise difficult system.
장점:
We set out to implement a QMS for our small Medical Device Company. To date, Greenlight Guru (GG) has proven to be an excellent choice. We are only a few months into the process but I see no problem in staying on track. Their support has been fantastic! We are ahead of schedule and I have been pleased with the quality of support we are getting. Our company has had a few false starts with consulting firms, GG is making the process enjoyable.
단점:
GG offers a package of procedure templates that make the job much easier. With that, the formatting and wording of the templates could be a tad more uniform. With that said, it is understandably difficult to make a universal set of templates that fit everyone's needs. I find the template package well done on that front.
Greenlight Guru 응답
6년 전
Hey Art - Our team greatly appreciates you taking the time to provide your feedback. We are thrilled to hear that our Customer Success team's Fanatical Support has jumpstarted your implementation process, while also making it an enjoyable experience. We appreciate your feedback regarding our procedure templates, as well. Please feel free to share your ideas for improvement with a member of our team. Thanks again - Glad to have you as a customer of Greenlight Guru!
Terrible customer service despite being super expensive
장점:
Good performance, good templates, comprehensive follow up for eQMS for med tech.
단점:
Too expensive, terrible customer service and attention.
Focus on the work - not the admin!
주석: It changed the way we documented risk related to the products. We streamlined our QMS and this helped us to come together as a team. Design reviews are easily assigned; change control tasks are set and executed - no more separate emails and phone calls to follow up - Greenlight does the nagging for you.
장점:
It sped up document control work flow and freed up the QA department's time to focus on validations and regulatory updates.
단점:
We can't think of anything at this time.
Greenlight Guru 응답
5년 전
Hey Dalene - Thanks for sharing your positive feedback about our software! It is great to hear that our software has freed up your team's time to focus more on strategic and value-add activities, instead of the manual labor of overseeing a QMS. If you ever have any questions or would like to provide us with more feedback, please do not hesitate to contact us.
Good to Great
주석: We really like using Greenlight guru and we believe that it is a service that all businesses in the medical industry should be using. However, when we signed on we were promised the addition of many various features. These features have not been added in the nearly two years we have been involved with Greenlight. I come from a software background and understand that things sometimes have to be placed on hold in order to fix more pressing issues. However, so far the additions that have been made have made greenlight more difficult to use rather than easier. We look forward to seeing some of the new features that have been promised to us in the near future.
장점:
It is the only Medical device management software on the market. Also, it allows us to centralize our numerous documents and other design records together in one place.
단점:
Limited functionality. We are still having to come up with our own solutions for numerous things that we believe were we able to use Greenlight it would immensely alleviate some of our work load.
Greenlight Guru 응답
6년 전
Hi, Douglas. It is great to work with you and your team. Since you left this comment, Greenlight Guru has added a ton of post-market functionality to help you better manage quality events. Now that you are using these features, how are things going?
Jeff Schwegman Review
주석: Overall my experience is positive. I like having the User Needs through Design Validation all on one dashboard in front of me. The Risk Management section is a little cumbersome to use at this point. I'm looking forward to new modules like ECO etc.
장점:
The dashboard showing User Needs; Design Inputs; Design Outputs; Design Verification; Design Validation all on one screen. I also like the Doc control capabilities
단점:
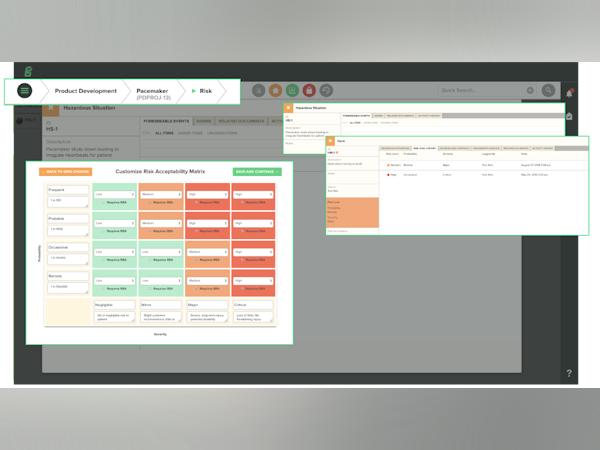
Risk Management section is cumbersome to use
Greenlight Guru 응답
6년 전
Hello Jeff, we appreciate you taking some time to write your review of our software. Your feedback regarding the risk management module has been heard and we believe you will be very excited about the changes coming with our risk 2.0 release. As it relates to your feedback regarding automatic notifications, we are pleased to share that this functionality is now available to further enhance your team's experience when routing documents through approval workflows. Glad to hear your overall experience has been positive and look forward to delivering on your feedback regarding the risk matrix.
Greenlight Guru does what it says on the tin....
주석: Green light Guru has been an invaluable resource not only in the set up an electronic QMS but in the advice available in the form of webinars and podcasts and someone who is new to the world of Quality and Regulatory requirements.
장점:
Aesthetically pleasing as well as practical and easy to use.
단점:
The risk and design modules have been difficult at times to use - the risk matrix is not easily maintained as a living record, e.g when a risk is mitigated and the scoring reduced.
Greenlight Guru 응답
8년 전
Hi Lizze, thank you very much for taking the time to review our software. We're glad to hear we've been able to live up to the promises made through our website and marketing. Your input, and the feedback we receive from all our customers is very important to us. That being said, we appreciate you comments regarding the risk matrix, and they have been added to our backlog. Your feedback will be considered as we look to release our risk 2.0 module.
FlexLogicals Review of Greenlight Guru's QMS Software
주석: We are saving a lot of time because it is so easy to link data to each other, and hence have the desired traceability. Furthermore, we are having a better overview of which requirements we have to verify and validate compared to our previous design matrix and risk matrix which was in Excel format. Lastly we are saving time during change control in general, this can be done within a few minutes while uploading a new verison of a document, technical drawing etc.
장점:
It is very easy to manage large amounts of data and have traceability on all of it. Especially during design & development, where many inputs have to be linked to outputs, verification(s) and validation(s). Furthermore, during the risk assessment the inputs and outputs, verifications and/or validations can easily be added as sources and controls to documents risk mitigation.
단점:
There is a lot of repeatable work while setting up a design matrix and risk matrix, but still faster than setting it up in an Excel Sheet.
Great solution for medical device companies
주석: GreenLight.guru has made implementing our quality system a manageable effort. We have likely saved half the effort and tens of thousands of dollars by using it for our first FDA submission.
장점:
The fact that it automates the process instead of just storing documents.
단점:
It would be great to have email notifications when you have an assigned activity.
Greenlight Guru 응답
6년 전
Hello Ross, thank you very much for taking the time to write your review. We really appreciate your input and feedback. We're happy to hear greenlight.guru has helped save you both time and dollars on your first FDA submission. Additionally, I am happy to share that automatic email notifications associated with tasks you've been assigned are now available in the platform.